Chemistry - Reactions - Redox Reaction
Category: Chemistry
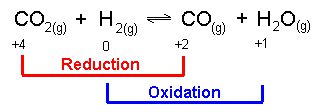
Image from UAB.EDU
In chemical reactions, "oxidation" is loosely defined as when a molecule, atom or ion loses an electron. "Reduction" is when a molecule, atom or ion gains an electron.
You can't have electrons just floating around at loose ends (it just isn't done!) and so "oxidation" and "reduction" always happen together and when they do, the resulting chemical reaction is called an "oxidation-reduction" reaction ("redox reactions" for short).
1. 2Na + Cl2
→
2. 2Na+ + 2e- + Cl2
→
3. 2Na+ + 2Cl-
→
4. 2NaCl
This reaction is the oxidation-reduction reaction that forms sodium chloride (NaCl) - the main ingredient (as in almost 100%) of table salt.It has been broken down into two "half-reactions" so you can see how (in oxidation) the electrons (e-) leave sodium (Na) and (in reduction) are picked-up by chloride (Cl). Note (step 3.) that the loss of electrons gives sodium a positive charge (Na+) and gaining electrons gives chloride a negative charge (Cl-). Since the sodium and chloride no longer have neutral charges, they are now defined as "ions". Because opposite charges attract, the positive sodium ions (2Na+) are attracted to the negative chloride ions (2Cl-) and so an ionic bond forms between them, making sodium chloride (NaCl) molecules. [An "ionic bond" is a chemical bond formed by the attraction between oppositely-charged ions]
REDOX IN COVALENT BONDS
Things are actually a bit more complicated because you can have "reduction" and "oxidation" even if no electrons are actually transferred.
Basically, you've been "oxidized" as long as you have less control over electrons than you did prior to the chemical reaction and you've been "reduced" as long as you have more control over electrons than you did before the chemical reaction occured.
So, even reactions that break and form covalent bonds (bonds where atoms share electrons instead of completely losing and gaining electrons), can qualify as "oxidation-reduction reactions". All that is needed is that someone has lost some control over their electrons and someone else has gained some control over their electrons.
"Reducing Agents" Oxidized and "Oxidizing Agents" Reduced!
To make things a little more confusing for beginning chemistry students, the substance that is "oxidized" is called the "reducing agent" because it has given up the electron(s) that the "reduced" substance needed in order to be "reduced". Similarly, the "reduced" substance is called the "oxidizing agent".

 Back To Category Chemistry
Back To Category Chemistry