Chemistry - Reactions - Acid-Base Reaction
Category: Chemistry
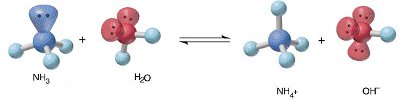
Image from UAB.EDU
Chemical compounds are both, stable and reactive. Generally the reactions in chemical compounds occur when there is more stability obtained in the process. All the compounds are formed due to the oxidation or reduction processes.
Many reactions that occur are as a result of either oxidation or reduction or both. According to the modern electronic concept, oxidation is the process of losing electron's and reduction is the process of gaining electrons. This can also be interpreted as the gain of a positive charge resulting in oxidation and the loss of a positive charge in reduction.
This means all electropositive elements get reduced when they transform from their compound metals. Similarly all the electronegative elements will get oxidized in their compounds and come to the elemental state. With this background one can conclude that the ionic compounds are a combination of an electropositive component and an electronegative component. The simplest electropositive component in water is a proton and the negative component is a hydroxide ion. Both combine to form H-O-H or H2O.
H+ is considered an acid component and OH- is considered a basic component. Together they form a neutral compound called water.
Acids are substances that are sour in taste and turn blue litmus red, methyl orange indicator in to red color and react with bases. They generally liberate hydrogen when treated with reactive metals. There are strong acids, weak acids, mono basic acids, di basic acids and tri basic acids. There are organic acids and inorganic or mineral acids. Strong acids have low pH, low pKa values and a high Ka value. Weak and less reactive acids will have lower values of Ka and high value of pKa. Their pH is around 5.0 - 6.5.
Bases are substances which turn red litmus blue and methyl orange to yellow. They are soap to touch and they react with acids to form salts. Water soluble bases are called alkalies. In modern definitions acids are the proton donors or a pair of electron acceptors, while bases are those which are proton acceptors or a pair of electron acceptors.
Acid base reaction can be defined as the reaction in which when equivalent quantities of are made to react a salt and water are formed. This reaction is called neutralization reaction. Thus a salt is a product other than the solvent formed when an acid and a base react.
ACID BASE NEUTRALIZATION REACTION
Acid base neutralization reaction is a reaction in which an acid reacts with a base to form salt and water. Poly functional acids and bases when react together there can be normal, acidic and basic salts that are formed. Normal salts are formed by the complete neutralization of acids and bases. When a multi protic acid is not completely neutralized by a base an acidic salt is formed. When a poly-functional base is incompletely neutralized by acid a basic salt is formed.
ACID BASE REACTION EXAMPLES
Hydrochloric acid combines in equivalent proportions with sodium hydroxide which is a base and forms a neutral salt sodium chloride and water. This reaction is represented by equation:
HCl + NaOH → NaCl + H2O
Equi molecular amounts of sulfuric acid and potassium hydroxide reacts with each other and forms an acidic salt potassium hydrogen sulphate. When one molecular mass of sulfuric acid and two molecular masses of potassium hydroxide are made to react a neutral salt potassium sulphate is formed. These reactions can be represented by the equations respectively.
H2SO4 + KOH → KHSO4 + H2O
H2SO4 + 2KOH → K2SO4 + 2H2O

 Back To Category Chemistry
Back To Category Chemistry