Chemistry - Bonds - Ionic Bonding
Category: Chemistry
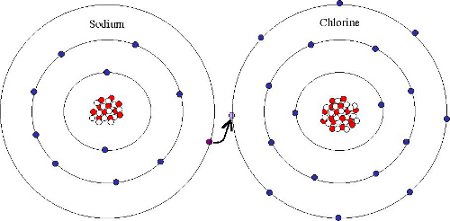
Ionic bonding involves transfer of an electron from one atom (which becomes a positively charged cation) to another (which becomes a negatively charged anion). The two ions attract strongly to form a crystal lattice.
Since ionic bonding requires that the atoms involved have unequal attraction for their valence electrons, an ionic compound must involve atoms of two quite different elements. Attraction for electrons depends on the distance of the electrons from the nucleus (which in turn depends on the amount of shielding by inner electrons). Ionic compounds generally form between metals toward the left and bottom of the periodic table, and nonmetals toward the right and top of the periodic table. The size of atoms increases as main shells are added going down groups, and decreases as electrons are added to the same shell across periods, because the increasing number of protons draws the outer shell closer. Metal atoms are large compared to nonmetal atoms with the same valence shell (in the same period)
The simplest example of a binary ionic compound is provided by the combination of elements number 1 (H) and number 3 (Li) in lithium hydride, LiH. On a microscopic level the formula LiH contains four electrons. In separate Li and H atoms these electrons are arranged as shown in part a of the following figure. The H atom has the electron configuration 1s^1, and Li is 1s^2 2s^1. When the two atoms are brought close enough together, however, the striking rearrangement of the electron clouds shown part b takes place. Here the color coding shows clearly that the electron density which was associated with the 2s orbital in the individual Li atom has been transferred to a 1s orbital surrounding the H atom. As a result, two new microscopic species are formed. The extra electron transforms the H atom into a negative ion or anion, written H- and called the hydride ion. The two electrons left on the Li atom are not enough to balance the charge of +3 on the Li nucleus, and so removal of an electron produces a positive ion or cation, written Li+ and called the lithium ion.
BOND STRENGTH
For a solid crystalline ionic compound the enthalpy change in forming the solid from gaseous ions is termed the lattice energy. The experimental value for the lattice energy can be determined using the Born-Haber cycle. It can also be calculated using the Born-Land'e equation as the sum of the electrostatic potential energy, calculated by summing interactions between cations and anions, and a short range repulsive potential energy term. The electrostatic potential can be expressed in terms of the inter-ionic separation and a constant (Madelung constant) that takes account of the geometry of the crystal. The Born-Land'e equation gives a reasonable fit to the lattice energy of e.g. sodium chloride where the calculated value is −756 kJ/mol which compares to −787 kJ/mol using the Born-Haber cycle.
POLARIZATION EFFECTS
Ions in crystal lattices of purely ionic compounds are spherical; however, if the positive ion is small and/or highly charged, it will distort the electron cloud of the negative ion, an effect summarised in Fajans' rules. This polarization of the negative ion leads to a build-up of extra charge density between the two nuclei, i.e., to partial covalency. Larger negative ions are more easily polarized, but the effect is usually only important when positive ions with charges of 3+ (e.g., Al3+) are involved. However, 2+ ions (Be2+) or even 1+ (Li+) show some polarizing power because their sizes are so small (e.g., LiI is ionic but has some covalent bonding present). Note that this is not the ionic polarization effect which refers to displacement of ions in the lattice due to the application of an electric field.
References:
Wikipedia.org
WISC.EDU

 Back To Category Chemistry
Back To Category Chemistry